Which Direction of the Reaction Is Favored at 298 K
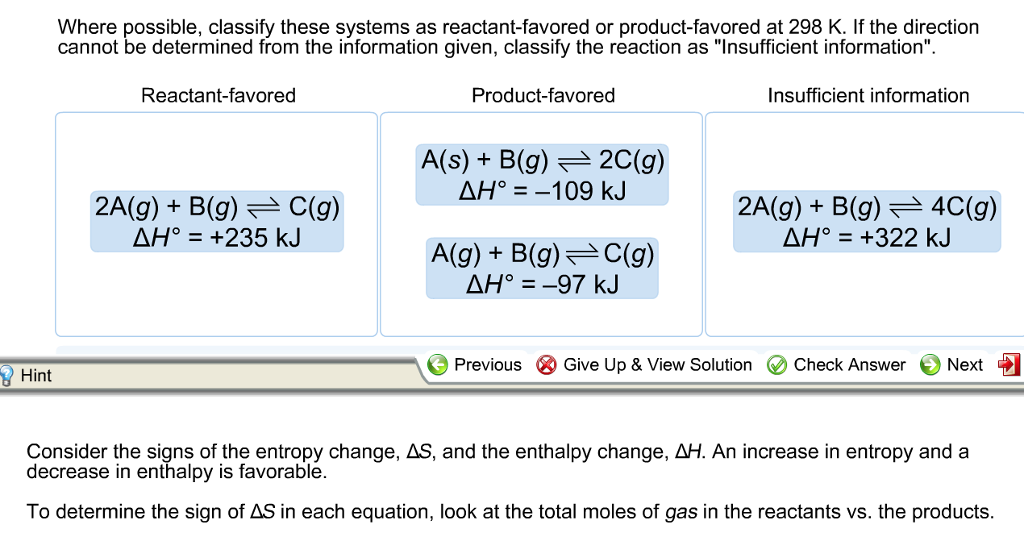
B2A g 2B g --3C g delta H 254kJ. 235 than on the right ie.
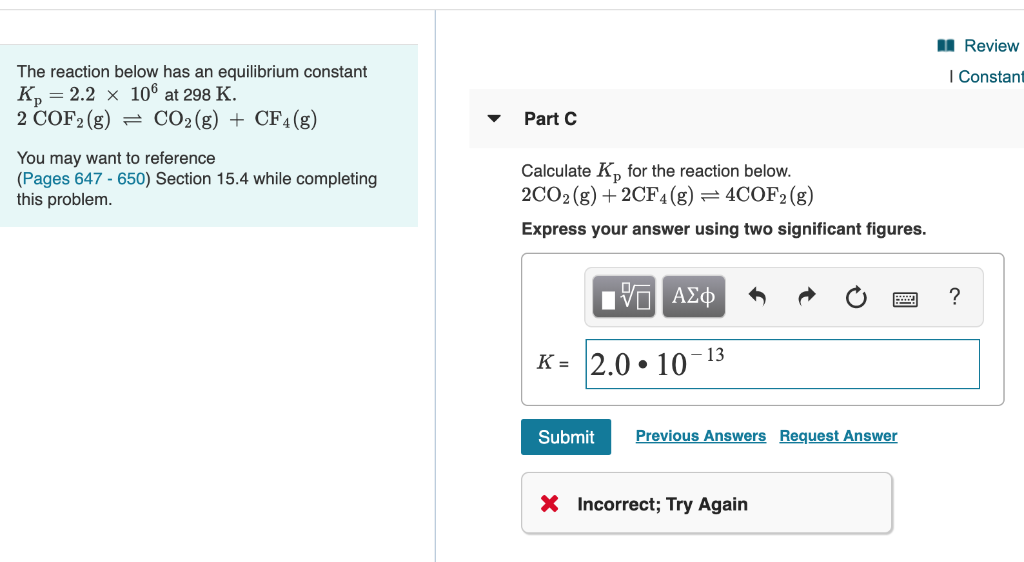
Solved The Reaction Below Has An Equilibrium Constant Chegg Com
SO2g ΔGf -300kJmol.

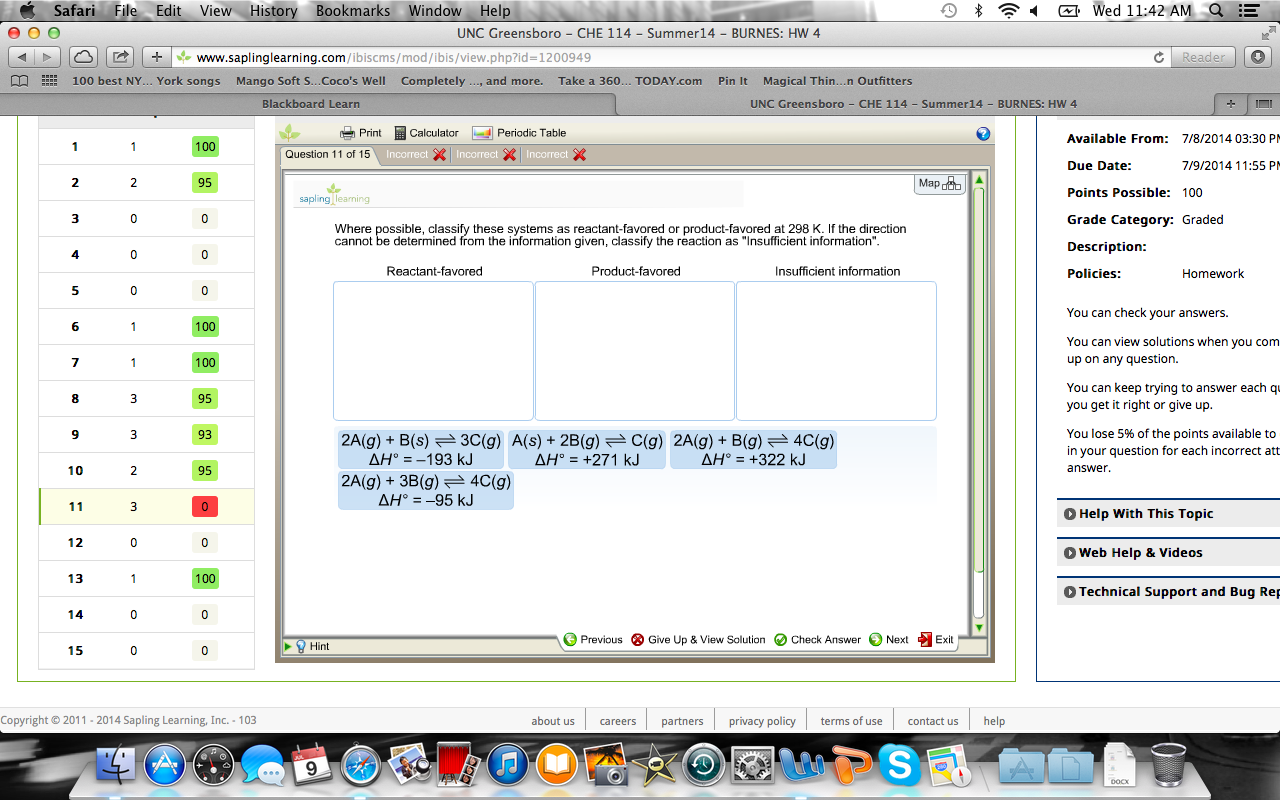
. If the direction cannot be determined from the information given classify the reaction as Insufficient information. Where possible classify these systems as reactant-favored or product-favored at 298 K. Which direction of the reaction is favored at 298 K room temperature.
Where possible classify these systems as reactant-favored or product-favored at 298 K. Answer this questi Toward I2s production on. Where possible classify these systems as reactant-favored or product-favored at 298 K.
Chemistry questions and answers. If its sign is - it means the reaction is spontaneous. Therefore the reaction will proceed in the forward direction producing more products until the concentrations reach their equilibrium values.
Reactant-favored Product-favored Insufficient information 2 A g 3 B g 4C g ΔΗ -95 kJ 2 A g B s 3 C g AH -193 kJ A g B g 3 C g Question. Answer this questi Toward I2s production on. Which direction of the reaction is favored at 298 K room temperature.
At 2300 K the equilibrium constant Kc is 17 x 10 -3 for the reaction. If the temperature is raised then the forward reaction is favored. Neither direction is favored.
Where possible classify these systems as reactant-favored or product-favored at 298 K. For the reaction described by the chemical equation. Which direction of the reaction is favored at 298 K room temperature.
The equilibrium constant for the given reaction is 00142 at 298 K. The ph of lemon juice at 298 k is found to be 232 what is the concentration oh H3O plus ions in the solution. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
2 SO2g O2g 2 SO3g An equilibrium mixture contains O2g and SO3g at partial pressures of 050 atm and 20 atm respectively. If the temperature is raised then the reverse reaction is favored. Using data from Appendix 4 determine the equilibrium partial pressure of SO2 in the mixture.
Where possible classify these systems as reactant-favored or product-favored at 298 K. G 624 kJmol - 298 K 0145 kJmolK G 1919 kJ. The ractions in which heat is either releasd H is negativeor absorbed H.
A2A g 3B g --4C g delta H -95kJ. DeltaH28 kJmol deltaS0109 kJmolK Which direction of the reaction equilibrium is favored at 298 K room temperature use the reaction I2 solid to I2 gas when H 624 kJmol and S 0145 kJmolK. Now the sign of G is a criterion for spontaneity.
If the direction cannot be determined from the information given classify the reaction as Insufficient information Reactant-favored Product-favored Insufficient information AO SO V 2 A B 3 C AH -193 S AH 2B 2 5CC AH. Answer 1 Reactant favored. Is this equilibrium reaction a product favored equilibrium with mostly products a reactant favored.
C2A g B g -- 4C g delta H 322 kJ. After 20 seconds NOBr 015 M NO 0020 M and Br2 0010. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
The reverse reaction is favored C Neither direction is favored. Is the reaction at equilibrium. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
This reaction is reactants-favored since on the left side we have greater entropy meaning there is a higher chance that reaction will happen spontaneously since there are more particles ie. Consider the following reaction at 298 K. Volume 220 L Molarity 120 M Temperature250C 273 298 K Decomposition reaction.
Since we got a positive sign that means the reaction is non-spontaneous. If the direction cannot be determined from the information given classify the reaction as Insufficient information Reactant-favored Product-favored Insufficient information AgB8 3g AH 388 AsBg 28 AH -109 2 AgB8 Cg AH 235 J 2 A8 3 Bg. Where possible classify these systems as reactant-favored or product-favored at 298 K.
3C2H2g - C6H6l. Where possible classify these systems as reactant-favored or product-favored at 298 K. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
If the direction cannot be determined from the information given classify the reaction as Insufficient information. Which direction of the reaction equilibrium is favored at 298 K room temperature use the reaction I2 solid to I2 gas when H 624 kJmol and S 0145 kJmolK. In these reaction CaCO3 is decomposed and forms the Calcium oxideCaO and the carbon dioxide.
Reactant-favored Product-favored Insufficient information BS 4C ДН--193 kJ 2Ag 2Bg-3Cg. Where possible classify these systems as reactant-favored or product-favored at 298 K. 2 Na2O2s Ss 2 H2Ol 4 NaOHaq SO2aq ΔH298 -610 kJmol.
Give the direction of the reaction if K. N2g O2g 2NOg. Consequently the reverse of this reaction would make it negative and spontaneous.
In the appendix I have the following. A2Ag 3Bg Chemistry. If not which way will the reaction proceed to reach equilibrium.
In the appendix I have the following. This is the best answer based on feedback and ratings.
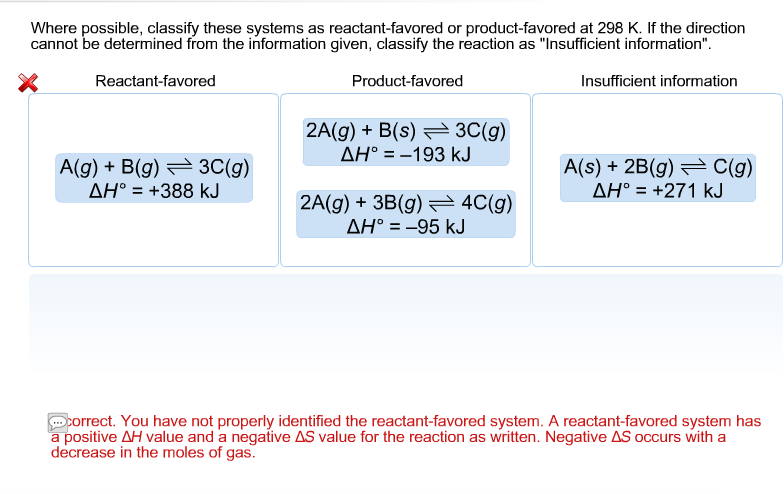
Solved Where Possible Classify These Systems As Chegg Com
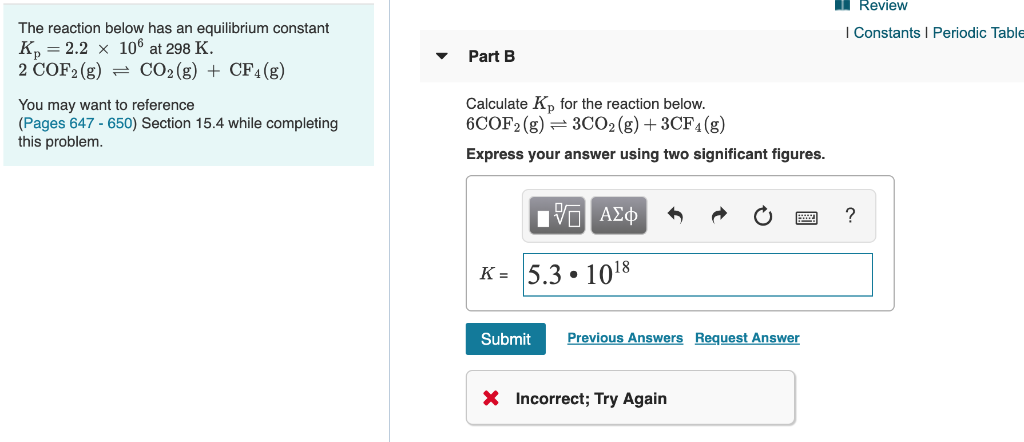
Solved The Reaction Below Has An Equilibrium Constant Chegg Com
Belum ada Komentar untuk "Which Direction of the Reaction Is Favored at 298 K"
Posting Komentar